Blog
Is Your Drug Ready for Preclinical Toxicological Testing?
Preclinical toxicological testing is a critical early milestone in drug development, acting as a gateway to clinical trials.
What Toxicity Data Is Needed Before First-In-Human (FIH) Trials?
To begin FIH trials, researchers have to demonstrate that the compound is reasonably safe for initial use in humans with toxicity data.
Oligos for Ocular Diseases: Overcoming the Challenges
Oligonucleotides, also known as “oligos,” are increasing in popularity due to their ability to target conditions previously thought undruggable.
7 Most Common Bioanalytical Testing Platforms for Small and Large Molecule Bioanalysis
Bioanalytical testing platforms are specific analytical tools that analyze and define the amount of drug molecules in biological fluid.
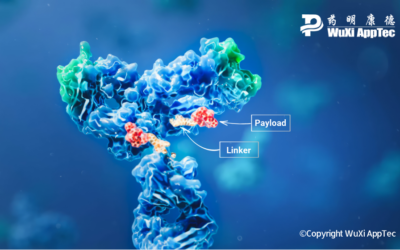
Striking the Right Balance: Linker Design in ADC Cancer Therapies
Single dose acute toxicity studies are small, relatively inexpensive studies – but critical to the success of your drug development program.
ELISpot Assays: The Key to Unlocking Advanced Cellular Immunity Testing
Single dose acute toxicity studies are small, relatively inexpensive studies – but critical to the success of your drug development program.
The Crucial Role of Biomarkers in the Precision Medicine Revolution
Single dose acute toxicity studies are small, relatively inexpensive studies – but critical to the success of your drug development program.
Acute Toxicity Studies: 3 Best Practices to Stay on Track with Your IND Timeline
Single dose acute toxicity studies are small, relatively inexpensive studies – but critical to the success of your drug development program.
Flux Dialysis: A Cutting-Edge Tool for PPB Studies & Understanding DDI
Metabolic stability is the susceptibility of compounds to biotransformation, which has a major impact on the efficacy and safety of drugs. Here are five assays that evaluate metabolic stability in the drug development process.
Metabolic Stability in Drug Development: 5 Assays
Metabolic stability is the susceptibility of compounds to biotransformation, which has a major impact on the efficacy and safety of drugs. Here are five assays that evaluate metabolic stability in the drug development process.
Repeat Dose Toxicity: 3 Possible Timeframes for Your IND-Enabling Studies
Repeat dose toxicity studies evaluate the effects of repeat administration over a defined period of time. To get the right toxicity data, what is the right duration for your compound? This blog discusses three possibilities. Toxicity data from repeat dose...
3 Must-Have Qualities of a Bioanalytical Method Development & Validation Partner
To make well-informed decisions about the future of your drug candidate, you need highly accurate and reliable data. Generating such data starts with bioanalytical method development and validation – a precise process that requires support from your testing partner....