Small Molecule Bioanalysis
Work with an expert team in analytical testing for cutting-edge small molecules. WuXi AppTec provides extensive capabilities and a state-of-the-art technology platform designed to streamline the advancement of your small molecule candidate to market efficiently.
Advanced Bioanalysis
for Small Molecules
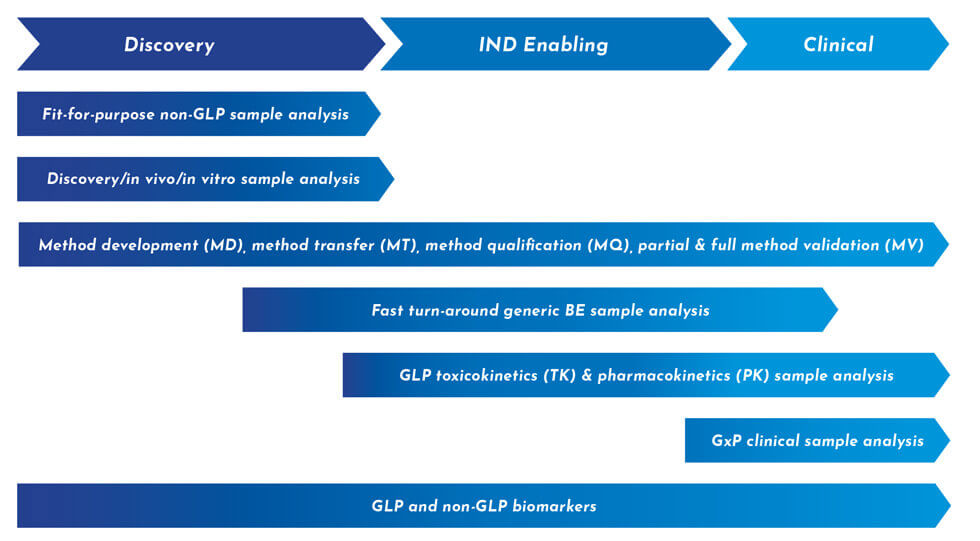
Our bioanalytical experts have decades of experience supporting and advancing small molecules through preclinical, IND-enabling and NDA. We can customize a testing program that addresses the specific bioanalytical challenges of your molecule and meets milestones on time.
-
200+ validated non-proprietary methods
-
40+ validated new methods for proprietary/innovator molecules every year
-
500,000+ sample analysis capacity
-
State-of-the-art mass spectrometry platforms with UHPLC-MS/MS capabilities
-
Automated and semi-automated high-throughput-capable systems including Janus®, TomTec® CyBio®-SELMA
-
21 CFR Part 11-compliant validated systems; Analyst®, MassLynx™, Watson LIMS™
Our Small Molecule Bioanalytical Experience
We are experts at delivering comprehensive bioanalytical programs for small molecules, including extensive experience with diverse small molecule compound classes, from common new chemical entities (NCE) to polar/non-polar and low molecular weight analytes, to endogenous analytes.
- 20+ years of experience in GLP/GCP bioanalysis
- 150+ novel compounds supported each year
- Experienced global project management team
- Vast bioanalytical capability of 500+ staff
- Global bioanalytical laboratory
- 200,000+ square feet
- Supported 2,000+ preclinical and clinical studies
- Currently collaborating with 200+ active clients
- Seamless method transfers between facilities to meet clients’ global clinical needs

Explore Our Small Molecule Bioanalytical Services
Your ability to make intelligent, informed decisions hinges on having the right bioanalytical strategy. We can help you develop an approach that will take you from discovery through preclinical to IND and beyond – with leading efficiency and speed.
Proven Expertise Across Species & Matrices
Our expertise extends across a wide range of animal species and matrices, providing precise and reliable analytical results. We adeptly tailor our methodologies to accommodate diverse biological contexts, ensuring accuracy and efficiency in bioanalysis.

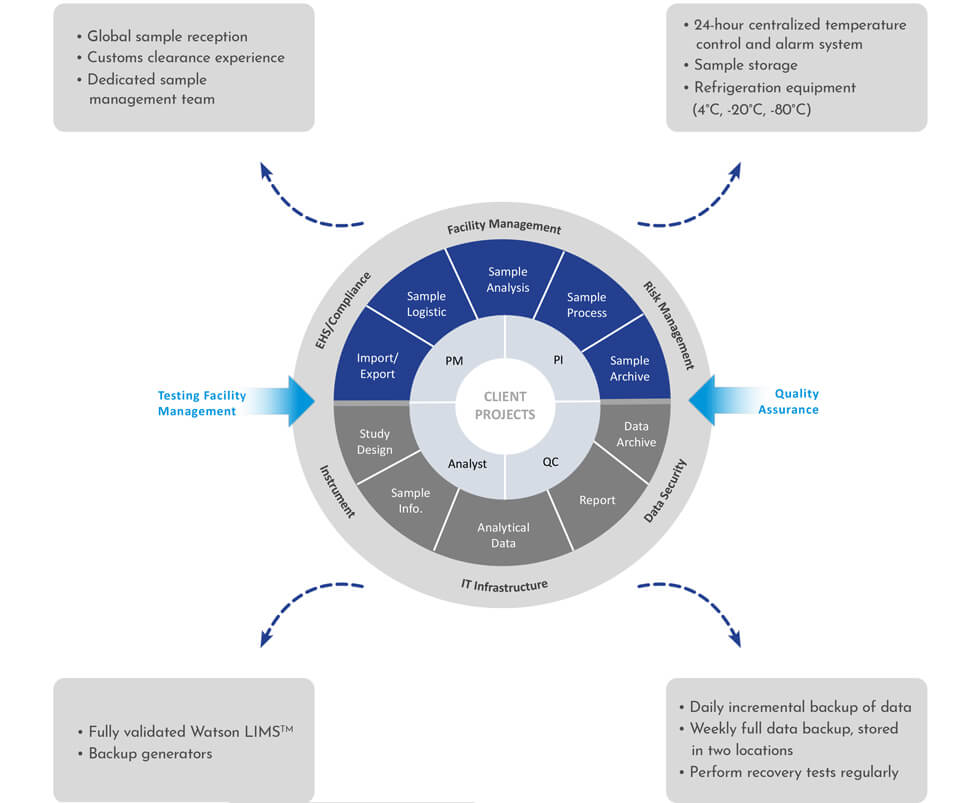
Comprehensive Quality Management
Our quality management system helps us ensure the highest standards in small molecule bioanalysis, with rigorous protocols and continuous oversight that guarantee accuracy, reliability, and compliance throughout every stage of the process.
LC-MS/MS Quantitation of Peptides & Proteins
We provide rapid method development and optimization for peptides and proteins, supported by extensive expertise and fast synthesis capabilities. Our services include handling diverse peptides and proteins across various species and matrices, utilizing advanced techniques for efficient and precise analysis.
Rapid Method Development
- Peptides 2-4 weeks
- Proteins 4-6 weeks
Extensive Experience
- Resolve non-specific binding
- Sensitivity tune-up
- Trypsin digestion conditions
- Microelution solid phase extraction (SPE) optimization
Diversified Peptides & Proteins
- Different species and matrices
- Hybrid Immunoaffinity LC-MS
Fast Synthesis Capabilities
- 2 weeks turnaround time
- Synthesize surrogate peptide
- Synthesize SIL-IS
Experience in Diverse Small Molecule Compound Classes
We have a breadth of experience and capabilities in developing and analyzing a wide range of small molecule compound classes, including innovative, challenging, and complex molecules.
- Common new chemical entities (NCE)
- Polar/non-polar and low molecular weight analytes
- Endogenous analytes
- Unstable analyte
- Amino acids & peptides
- Insulin analogs
- Biomarkers
- Vitamins
- Lipids
- Oligonucleotides
- Antibody-drug conjugates
Regulatory Support
Get broad and deep regulatory guidance and support throughout every stage of your development process.
- GLP/non-GLP bioanalytical facilities
- Central laboratory services – safety lab and clinical kits
- Support for innovator and generic drugs
- Clinical and preclinical study support
- Submitted data accepted by worldwide health authorities
- Completed FDA, EMA, PMDA, NMPA (CFDA) study inspections with minor observations and no deficiencies
- Completed multiple GLP inspections and certifications by OECD
- Successfully complete 50+ client inspections each year
- Drug Enforcement Agency-inspected, state licensed for radioactivity use (U.S.)
State-of-the-Art Instrumentation
Our cutting-edge facilities are stocked with some of the most advanced bioanalytical equipment in the world.
- 70+ UPLC™-MS/MS instruments globally
- SCIEX API Triple Quad™
- 4000/5000/5500/6500/6500+
- Waters Xevo® TQ-S
- Waters UPLC™/Shimadzu HPLC/Agilent HPLC/Shimadzu UHPLC
- Fully automatic LC method scouting system
- PerkinElmer Janus® liquid handling system
- TomTec® liquid handling system
Small Molecule Bioanalytical FAQ
What is small molecule bioanalysis?
The analysis of small molecules involves accurately measuring the levels of low molecular weight compounds, including drugs and their byproducts, in different biological samples. This is essential for assessing small molecule treatments’ effectiveness, safety and pharmacokinetics.
Why is bioanalytical testing important for small molecules?
Studying the bioanalytical aspects of small molecules is crucial for gaining insights into their absorption, distribution, metabolism and excretion (ADME) properties. This knowledge is instrumental in determining the appropriate dosage, evaluating therapeutic effectiveness and safeguarding patient well-being.
What types of samples are analyzed in small molecule bioanalysis?
Various types of samples, such as blood, plasma, serum, urine, tissue, and other biological fluids, are examined to measure the levels of small molecule drugs and their metabolites.
What types of samples are analyzed in small molecule bioanalysis?
Various types of samples, such as blood, plasma, serum, urine, tissue, and other biological fluids, are examined to measure the levels of small molecule drugs and their metabolites.
What analytical techniques are used in small molecule bioanalysis?
In small molecule bioanalysis, commonly utilized techniques include liquid chromatography-mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC). These methods offer high sensitivity and specificity in detecting and quantifying analytes.
How are bioanalytical methods validated for small molecules?
Small molecule bioanalytical methods are validated in accordance with regulatory guidelines to guarantee accuracy, precision, sensitivity, specificity, reproducibility and robustness. Validation is essential to ensure that the methods consistently yield reliable and consistent results.
Can customized bioanalytical services be provided for small molecule projects?
Yes, tailored bioanalytical services can be customized to suit the specific requirements of various small molecule projects. This involves creating and validating distinct analytical methods to tackle particular research inquiries.
What are the challenges in small molecule bioanalysis?
Overcoming challenges in small molecule bioanalysis necessitates addressing low analyte concentrations, matrix effects in complex biological samples and the need for high sensitivity and specificity. Experienced scientists and advanced techniques play a crucial role in conquering these obstacles.
How long does it typically take to complete a small molecule bioanalysis study?
The length of a small molecule bioanalysis study varies depending on the project’s complexity, sample quantity and necessary analytical techniques. Typically, timelines are established and agreed upon during the project planning phase.
What services does WuXi AppTec offer for small molecule bioanalysis?
WuXi AppTec provides comprehensive small molecule bioanalytical services tailored for drug development, from study design to regulatory submission. Our services include a selection of standard assays and a team that can develop custom biomarkers from method development through validation, including high-throughput sample analysis.
What technologies are used in WuXi AppTec's small molecule bioanalysis?
The laboratory utilizes state-of-the-art mass spectrometry platforms, including UHPLC-MS/MS and automated and semi-automated 21 CFR Part 11-compliant systems.
Can WuXi AppTec handle high volumes of sample analysis for small molecules?
Yes, the facilities can analyze more than 500,000 samples and routinely develop more than 40 new methods for proprietary molecules annually.
Does WuXi AppTec support regulatory submissions for small molecule studies?
Yes, we offer integrated bioanalytical services that support innovator and generic drugs for global regulatory submissions, including studies for clinical and preclinical phases.
What types of compounds and matrices can WuXi AppTec analyze?
WuXi AppTec has extensive experience with diverse small molecule compound classes and biological matrices, supporting studies across various species.
Where are WuXi AppTec’s bioanalytical labs located?
We have leading integrated laboratory operations in New Jersey and China, including Central Laboratory Services.