Drug Metabolism and Pharmacokinetic Services
Speak With a Scientist
about your upcoming project to see how we can help you
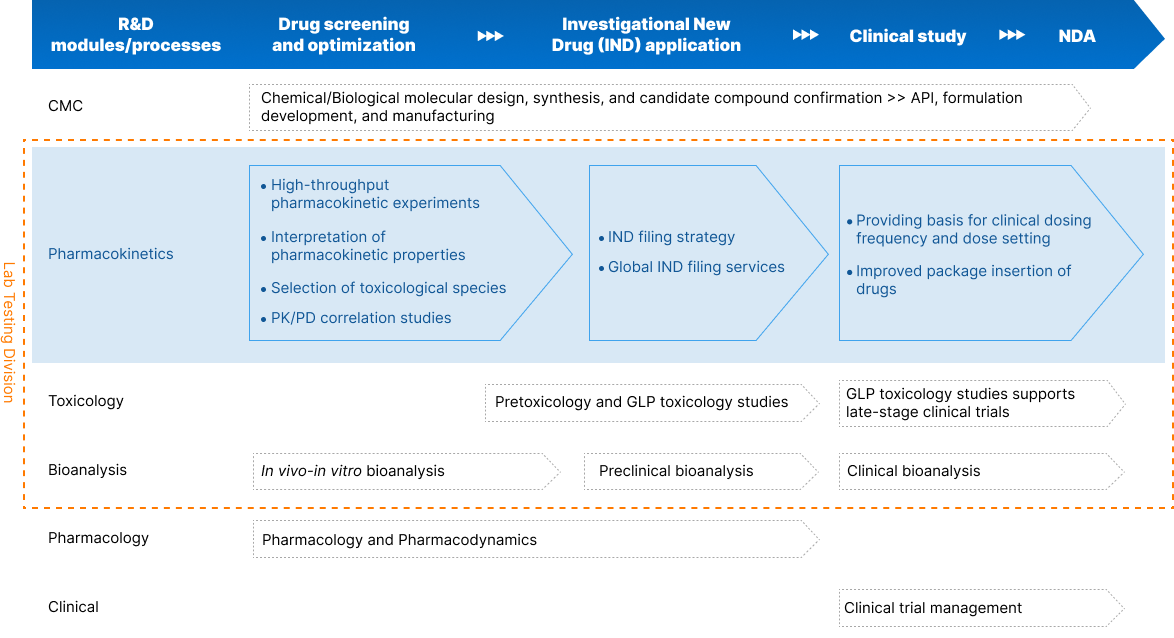
Global DMPK/ADME Studies
Shifting the focus of DMPK/ADME studies to an earlier stage in the discovery process can play a pivotal role in reducing the likelihood of compound failure. This strategic realignment not only aids in establishing compound potency against a target while ensuring safety but also fine-tunes PK parameters to enhance effectiveness. Additionally, it mitigates the potential for drug-drug interactions (DDIs). Subsequently, during later stages, ADME studies further validate toxicological assessments, provide crucial backing for safety evaluations before First-in-Human (FIH) trials, and offer insights into the probability of encountering DDIs.
Integrated Drug Metabolism & Pharmacokinetics
Effective DMPK/ADME studies require a full understanding of your specific needs and challenges. This allows our team to build a program tailored to the needs of your compound and timeline. Whether providing stand-alone or integrated services, our DMPK/ADME team collaborates closely with our chemistry, biology and pharmacology groups to support your compound’s design and selection during the discovery stage.
Years Experience
DMPK/ADME Employees
Global Clients
Successful IND Filings
Assay Types
In Vitro Studies/Year
In Vivo Studies/Year
Samples Analyzed/Week
Comprehensive DMPK/ADME Services
Our DMPK services range from expert in vitro ADME and in vivo PK to full-scale digital lab operation and management. We combine a full complement of services and expertise and open, transparent communication between teams and specializations, helping your project advance seamlessly through the life cycle.
In vitro ADME
In vitro ADME studies analyze how potential drugs are absorbed, distributed, metabolized, and excreted (ADME) within a controlled environment to predict efficacy, safety, and potential interactions, and reduce development costs.
- Physicochemical property
- Permeability
- Whole blood/plasma distribution and protein binding
- Matrix and metabolic stability
- Drug-drug interactions (DDIs) mediated by drug-metabolixing enzymes and transporters


In vivo PK
In vivo PK studies evaluate a drug’s pharmacokinetics (PK) to provide vital data on how the body processes a drug. This helps assess its efficacy, safety, and optimal dosing regimens during the drug development process.
- Rodent in vivo PK and non-rodent in vivo PK
- Preclinical vehicle screening
- PK/PD correlation
- Animal facilities accredited by AAALAC and OLAW
Metabolite Identification (MetID)
Metabolite identification examines the breakdown products of a drug—metabolites—within a biological system, helping understand their potential effects on a drug’s overall efficacy and safety profile.
- Soft spot and reactive metabolites
- Metabolite identification (IND/Preclinical)
- Metabolite identification in human and MIST studies
- Metabolite identification and radioprofiling
- Metabolite biosynthesis and structural characterization
Radiolabeled ADME
Radiolabeled ADME studies provide insights into a drug’s journey in the body, helping evaluate its behavior, potential accumulation, and elimination routes, which inform later-stage decision-making.
- Radiolabeled compound synthesis
- Radiolabeled rodent and non-rodent study in mass balance, QWBA and metabolite identification
- Human AME (hAME) studies
Non-GLP Bioanalysis
Non-GLP bioanalysis involves evaluating drugs, metabolites, or biomarkers in biological matrices in a laboratory setting that does not adhere to the regulatory guidelines of Good Laboratory Practice (GLP)—providing valuable early data for drug discovery and development.
- Analytes include small molecule, PROTAC, oligonucleotide, antibody, ADC, peptide, recombinant proteins, mRNA, biomarker, etc.
- Analytical platforms include LC-MS, ELISA, MSD, qPCR, RT-qPCR, etc.


Digital Lab Operation
Our digital lab operation services help streamline the management, storage, and logistics needs of your project to optimize workflows, minimize error, and ensure efficient and safe data delivery.
- Compound management
- Whole-process digital operation
- QuickTracer online project tracking
DMPK/ADME Studies for Your Molecule
Our expert DMPK/ADME team can support a wide range of molecules and compounds, including new modalities. We offer a variety of study packages, from Investigational New Drug (IND)-Enabling to Definitive Drug-Drug Interaction (DDI) to Biotransformation and In Vivo Absorption, distribution, Metabolism and Excretion (ADME).
- Small molecules and challenging chemical drugs
- PROTAC
- Oligonucleotide
- Antibody Drug Conjugate (ADC)
- Peptide Drug Conjugate
- Peptide
- mRNA
- Bispecific antibody
- New structure antibodies
- New delivery systems
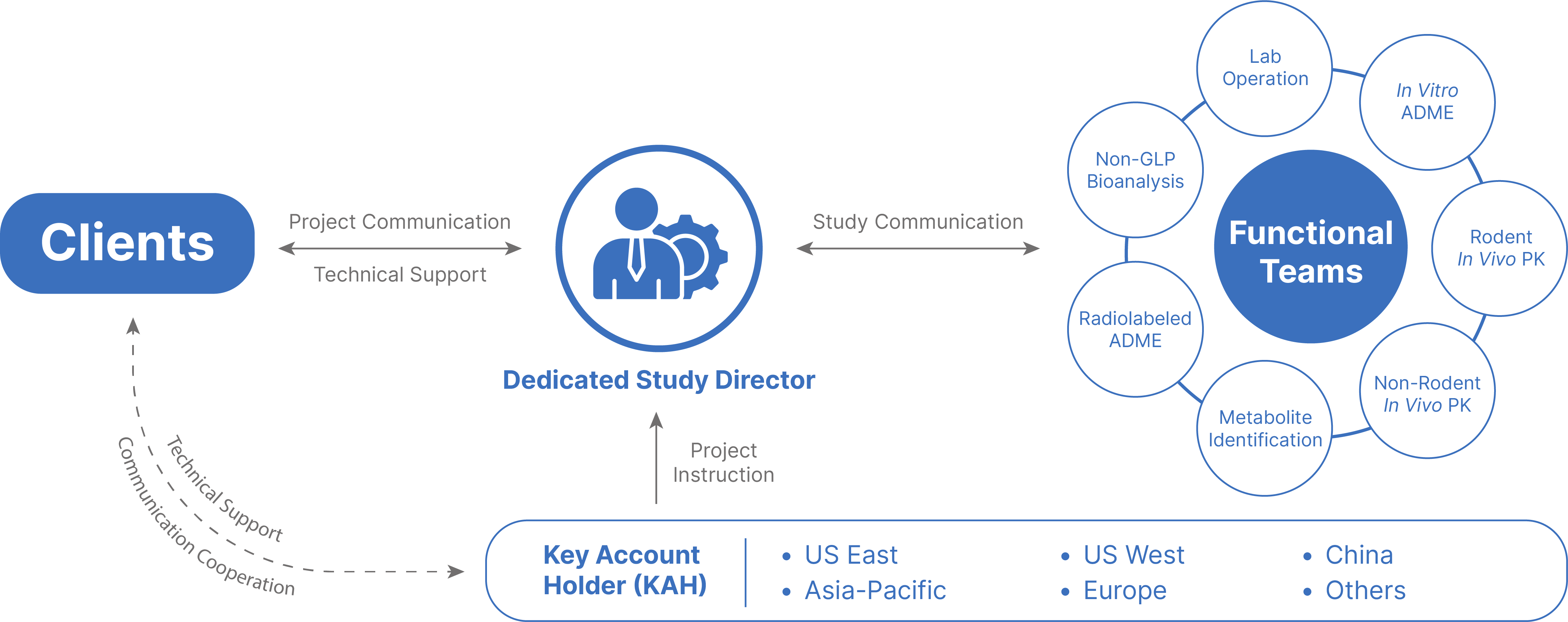
Our DMPK/ADME Network
Gain access to an integrated, worldwide network of facilities, including five R&D centers across China and the United States. We offer a full range of discovery screening, preclinical development and clinical drug metabolism and pharmacokinetic platforms and services.
Why WuXi AppTec?
Trusted by more than 1,500 customers globally, WuXi AppTec is a global leader in preclinical drug development testing. Our DMPK/ADME programs provide comprehensive service, impeccable standards and quality, and fast turnaround—all backed by years of extensive experience.
Comprehensive Service
- Screening to IND filing
- In vitro and in vivo
- Routine small molecules and new modalities
Fast Data Delivery
- No lead time for in vitro and in vivo assays
- Less than five working days for screening assays
- 4 to 5 months for regular IND-enabling package
State-of-the-Art Instruments & Facilities
- AAALAC accredited & OLAW compliant
- FDA, EMA, EPA, NMPA, USDA, DEA compliant
- State-of-the-art bioanalytical instruments
Experienced & Professional Teams
- Highly experienced professional teams
- 15+ years of experience
- 1,000+ DMPK/ADME professionals
High Throughput Automation Platform
- Fully automated in vitro ADME workstation
- Fully automated bioanalysis sample pretreatment system
Reliable Experimental Results
- Meet IND and NDA requirements of FDA, NMPA and EMA 15+ years of surveillance/audit
- 150+ NMPA inspections
Frequently Asked Questions
What are DMPK/ADME services?
DMPK/ADME services help support drug developers in understanding the Absorption, Distribution, Metabolism, and Excretion (ADME) processes of specific compounds or drugs. DMPK services include the study of pharmacokinetics.
What is the difference between ADME and DMPK?
ADME is a subset of DMPK, focusing on the absorption, distribution, metabolism, and excretion of drugs. In addition to ADME, DMPK adds pharmacokinetics including considerations of dosage regimens, optimal dosage frequencies, and factors affecting drug efficacy and safety.
Why are DMPK/ADME studies important in drug discovery?
DMPK/ADME studies are crucial in evaluating the pharmacokinetic behavior of potential drug candidates, optimizing dosing regimens, and bridging their safety and efficacy before advancing to later stages of development.
What kind of testing do DMPK/ADME studies include?
DMPK/ADME studies include a range of in vitro and in vivo assays to understand how the body interacts with a drug or compound. To be more specific, these studies include in vitro ADME (Absorption, Distribution, Metabolism, and Excretion), rodent pharmacokinetics, large animal pharmacokinetics, metabolites profiling and identification, mass balance and tissue distribution, etc.
How does DMPK/ADME testing add value to my program?
When you partner with WuXi AppTec for drug discovery and development services, you gain access to an integrated worldwide network of facilities that offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. DMPK/ADME testing will run through the entire process of drug discovery, preclinical development, IND enabling and clinical studies.
Are WuXi AppTec facilities compliant?
Yes, our DMPK/ADME facilities are compliant with the U.S. Food and Drug Administration (FDA), European Medical Agency (EMA), Environmental Protection Agency (EPA), National Medical Products Administration (NMPA), U.S. Department of Agriculture (USDA) and Drug Enforcement Administration (DEA) regulations. They are also fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and compliant with the Office of Laboratory Animal Welfare (OLAW) Assurance to Conduct Public Health Service (PHS)-funded studies.