Large Molecule Bioanalysis
Work with a team of experts at the forefront of analytical testing for the next generation of biotherapeutics. WuXi AppTec offers comprehensive capabilities and a sophisticated technology platform dedicated to clearing a path to more efficiently advance your biotherapies to market.
Industry-Leading Bioanalysis
for Large Molecules
WuXi AppTec’s bioanalytical team has the experience and expertise to help you move your drug candidate to the next milestone. We’ll customize a program that meets your project’s evolving needs and supports you from preclinical through new drug application (NDA).
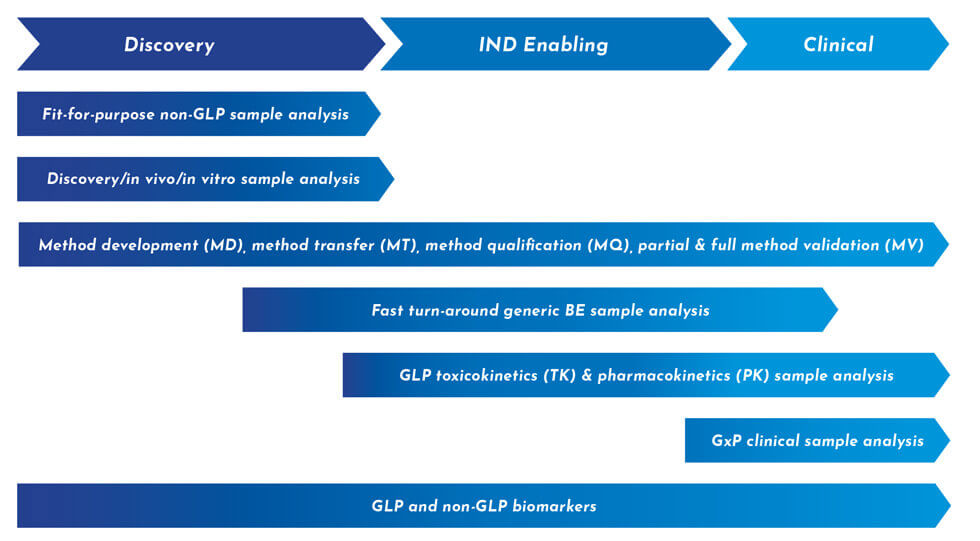
- Pharmacokinetics (PK) Bioanalytical Services
- Toxicokinetics (TK) Bioanalytical Services
- Immunogenicity ADA and Neutralizing Antibody (NAb) Service
- Statistical analysis of Cut-Point and Low Positive Control determination
- Exploratory and decision-making biomarker screening/discovery: Ligand Binding Assay (LBA) and LC-MS/MS Services
- Monoclonal and Polyclonal Reagent Generation
Our Large Molecule Bioanalytical Experience
We are experts at delivering advanced bioanalytical programs customized to a specific biotherapy. Our team has years of experience executing validated assays across a range of testing platforms.
Representative Large Molecule Programs

Explore Our Large Molecule Bioanalysis Services
Your ability to make intelligent, informed decisions hinges on having the right bioanalytical strategy. We can help you develop an approach that will take you from discovery through preclinical to IND and beyond – with leading efficiency and speed.
Immunogenicity Bioanalytical Services (GLP)
Biological drugs pose unique challenges during development, particularly their higher likelihood of triggering an immune response compared to small molecule drugs, which can result in adverse effects. Regulatory agencies mandate comprehensive immunogenicity testing, and paying attention to this critical component in your IND package can help prevent significant clinical delays. At WuXi AppTec, we partner with our clients to develop tailored reagents and assays that assess and predict key immune response markers. Our expert team also provides comprehensive support in implementing multi-tiered testing strategies that comply with the latest regulatory immunogenicity guidelines.
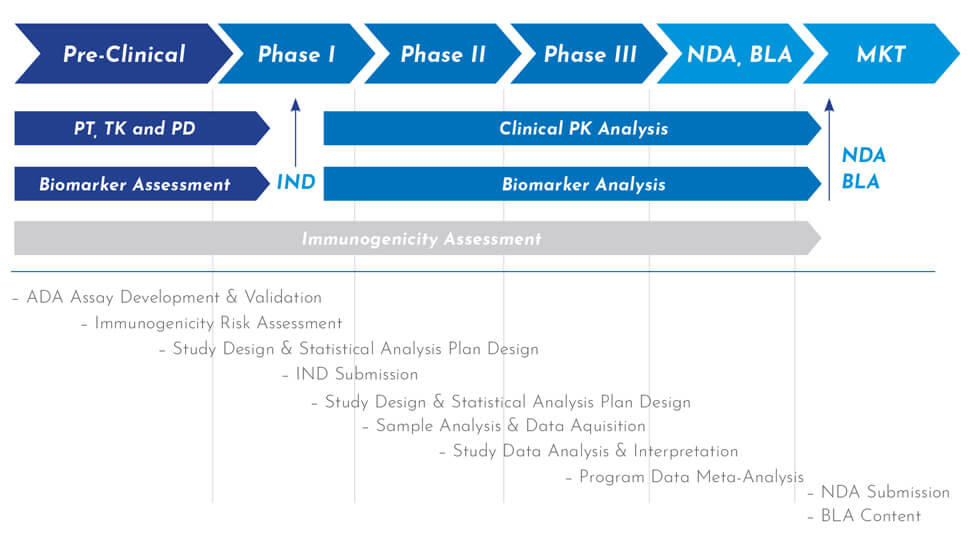
Screening Confirmatory & Titer Determination
We’re experts in the screening, confirmatory, and titer determination processes for biological therapeutics. We provide advanced assays, statistical analysis, and precise results, ensuring high drug tolerance and compliance with FDA standards.
- Reagent generation to fit biological therapeutic using monoclonal or polyclonal antibodies for positive controls and epitope-specific reagent purification
- Anti-drug antibody (ADA) electrochemiluminescent (ECL) bridging assays with capabilities of executing complex assays for difficult biotherapeutics
- Statistical analysis of cut-point and low positive controls in U.S. FDA-acceptable report format
- Non-cell-based neutralizing antibody (NAb) assays
- High drug tolerance with bead extraction and acid dissociation (BEAD)
- Cell-based neutralizing antibody assays

Regulatory Support
Get broad and deep regulatory guidance and support throughout every stage of your development process.
- GLP/non-GLP bioanalytical facilities
- Central laboratory services – safety lab and clinical kits
- Support for innovator and biosimilar drugs
- Clinical and preclinical study support
- Submitted data accepted by worldwide health authorities
- Completed FDA, EMA, PMDA, NMPA (CFDA) study inspections with minor observations and no deficiencies
- Completed multiple GLP inspections and certifications by OECD
- Successfully complete 50+ client inspections each year
- Drug Enforcement Agency-inspected, state licensed for radioactivity use (U.S.)
Large Molecule Bioanalytical FAQ
What is large molecule bioanalysis?
Quantitative analysis of large molecules involves measuring biological drugs, including proteins, peptides, and monoclonal antibodies, in different biological samples. This method is crucial for assessing the pharmacokinetics, effectiveness and safety of large molecule treatments.
Why is bioanalytical testing important for large molecules?
Testing large molecules through bioanalysis is crucial because of their intricate structures and distinctive pharmacokinetic characteristics. Precise measurement is essential for scientists to understand their absorption, distribution, metabolism, and excretion, which is vital for optimizing dosage and conducting safety assessments.
What types of samples are analyzed in large molecule bioanalysis?
Large molecule samples usually consist of blood, plasma, serum, urine, tissue and other biological fluids. They are examined to measure the levels of medications and their byproducts.
What analytical techniques are used for large molecule bioanalysis?
Commonly employed techniques for large molecule bioanalysis include ligand-binding assays (LBAs) like enzyme-linked immunosorbent assay (ELISA), electrochemiluminescence (ECL) and surface plasmon resonance (SPR). In addition, mass spectrometry-based methods (e.g., LC-MS/MS) are utilized for comprehensive analysis.
How are bioanalytical methods validated for large molecules?
Bioanalytical techniques for large molecules undergo validation in compliance with regulatory standards to guarantee accuracy, precision, sensitivity, specificity and reproducibility. Validation serves to verify that the techniques yield dependable and uniform results.
What regulatory guidelines govern large molecule bioanalysis?
Bioanalysis of large molecules must adhere to regulatory guidelines set forth by international regulatory bodies. These guidelines delineate the criteria for validating methods, analyzing samples, and reporting data.
Can customized bioanalytical services be provided for large molecule projects?
Yes, bioanalytical services can be customized to cater to the specific requirements of various large molecule projects. This encompasses developing and validating distinctive analytical methods to tackle particular research inquiries.
What are the challenges in large molecule bioanalysis?
Challenges arise from large molecules’ intricate structure and size, their potential immunogenicity, and the requirement for highly sensitive and specific analytical methods. Overcoming these challenges is made possible with advanced techniques and the expertise of experienced scientists.
How long does it typically take to complete a large molecule bioanalysis study?
The timeframe for a large molecule bioanalysis study is contingent upon the project’s complexity, the quantity of samples, and the analytical methods needed. Typically, timelines are deliberated and finalized during the project planning phase.
What types of large molecule bioanalytical services does WuXi AppTec provide?
WuXi AppTec offers a range of large molecule bioanalytical services, including pharmacokinetics (PK), toxicokinetics (TK), immunogenicity testing, and biomarker discovery. These services support drug development from discovery to regulatory submissions, including extensive experience with new modalities.
What technologies are employed in large molecule bioanalysis at WuXi AppTec?
The lab utilizes advanced technologies such as Ligand Binding Assays (LBA), LC-MS/MS, and cell-based assays to ensure precise and reliable results.
Can WuXi AppTec customize bioanalytical studies for specific project needs?
Yes, WuXi AppTec tailors its bioanalytical programs to meet the evolving needs of each project, ensuring efficient and practical advancement of drug candidates.
How does WuXi AppTec handle immunogenicity assessments for large molecules?
The company provides comprehensive immunogenicity testing, including anti-drug antibody (ADA) and neutralizing antibody (NAb) assays, adhering to U.S. regulatory guidelines.
What support does WuXi AppTec offer for regulatory submissions involving large molecules?
WuXi AppTec supports all stages of regulatory submission, providing expert guidance and customized testing to meet stringent regulatory standards.
Where are WuXi AppTec’s bioanalytical labs located?
We have leading integrated laboratory operations in New Jersey and China, including Central Laboratory Services.