In Vitro Toxicology
In Vitro Toxicology Testing involves evaluating the potential toxicity of substances using cells or tissues outside the body to predict their effects on humans. WuXi AppTec’s Genetic and In Vitro Toxicology (GIVT) department offers a robust platform for in vitro electrophysiology and phototoxicity testing and evaluation. With New Approach Methodologies (NAMs) gaining increasing attention, we are devoted to explore more NAMs combining in silico, in chemico and in vitro under this platform.
Years of In Vitro Toxicology Experience
Safety Assessment Staff
Safety Assessment Studies Conducted/Year

The state-of-the-art laboratory and instruments.
In Vitro Electrophysiology Testing & Evaluation
When ventricular repolarization is delayed and the QT interval is prolonged, there is an increased risk of ventricular tachyarrhythmia, including torsade de pointes (TdP). Prolongation of the QT interval can result from the inhibition of the hERG potassium channel current and the effect can be weakened by the inhibition of sodium and calcium channel currents.
The comprehensive in vitro proarrhythmia assay (CiPA) is a new strategy that can predict QT interval prolongation risk through a battery of nonclinical safety evaluation assays, including multi-ion channel assays, computational modeling assay, stem cell-derived cardiomyocytes assay and clinical testing. These assays have an important role in assessing the potential for QT interval prolongation and are recommended by regulatory agencies.
To date, WuXi AppTec offers multi-ion channel assays. We are focusing on developing computational modeling and stem cell-derived cardiomyocytes assays.
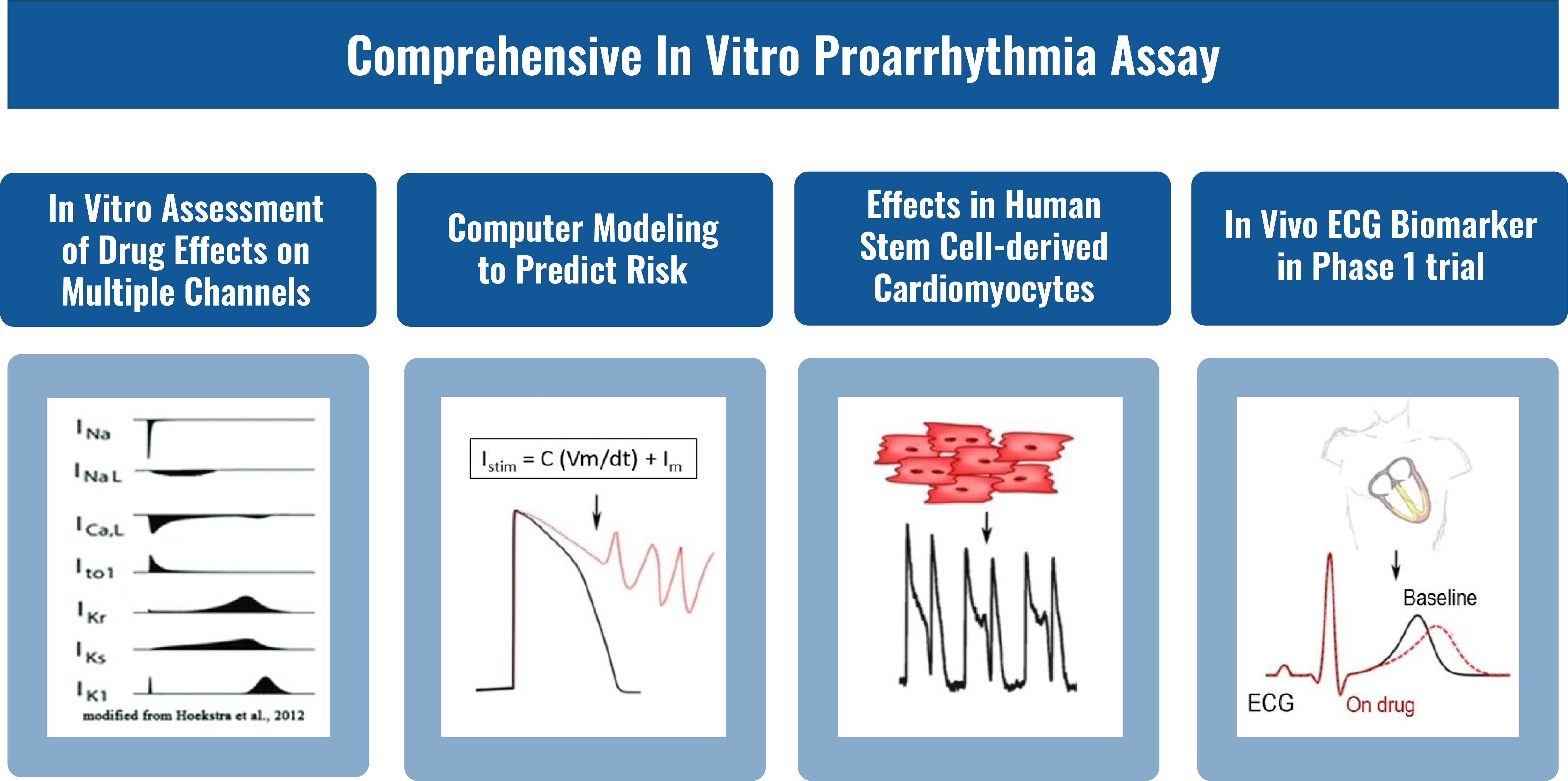
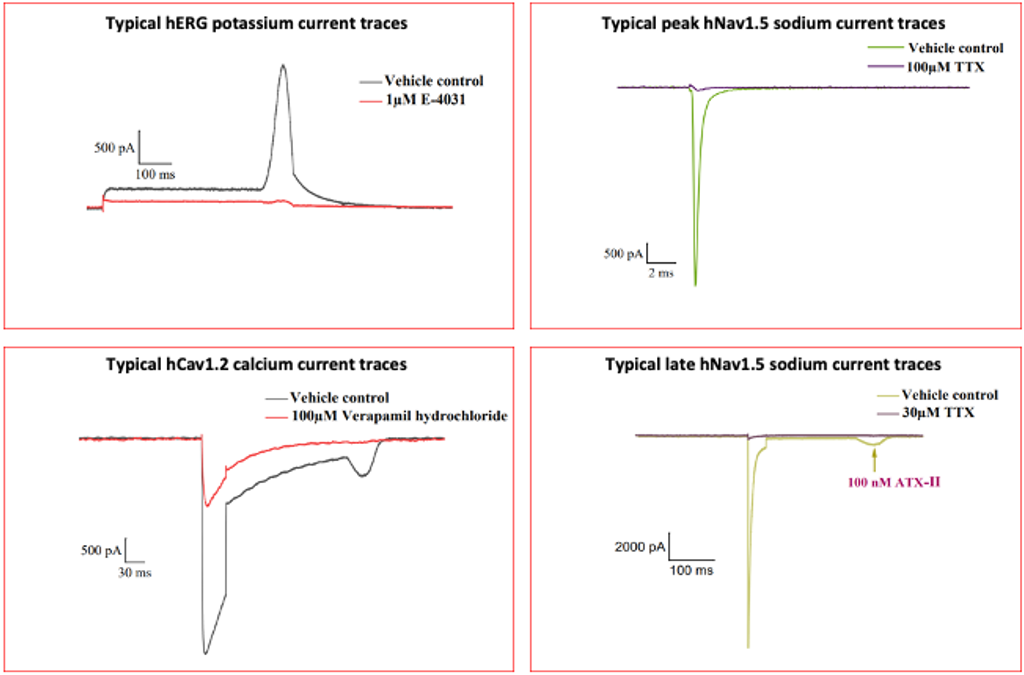
GLP or Non-GLP In Vitro Electrophysiology Assay
In vitro electrophysiology assays provide crucial insights into the electrical activity of compounds, helping to assess their potential impact on cardiac ion channels and overall safety profiles. WuXi AppTec’s standard battery of electrophysiology assays include both GLP and non-GLP assays:
- hERG assay: Evaluates the potential of compounds to block the hERG potassium ion channel.
- Peak hNav1.5 assay: Measures the effects of compounds on the peak sodium current through the hNav1.5 channel, involved in cardiac action potentials.
- Late hNav1.5 assay: Assesses compounds’ effects on the late sodium current through the hNav1.5 channel, relevant for understanding arrhythmia risks.
- hCav1.2 assay: Examines compounds’ interactions with the hCav1.2 calcium ion channel, involved in cardiac action potentials.
Phototoxicity Testing & Evaluation
Phototoxicity is defined as a toxic response elicited by topically or systemically administered photoreactive chemicals after the body’s exposure to environmental light.
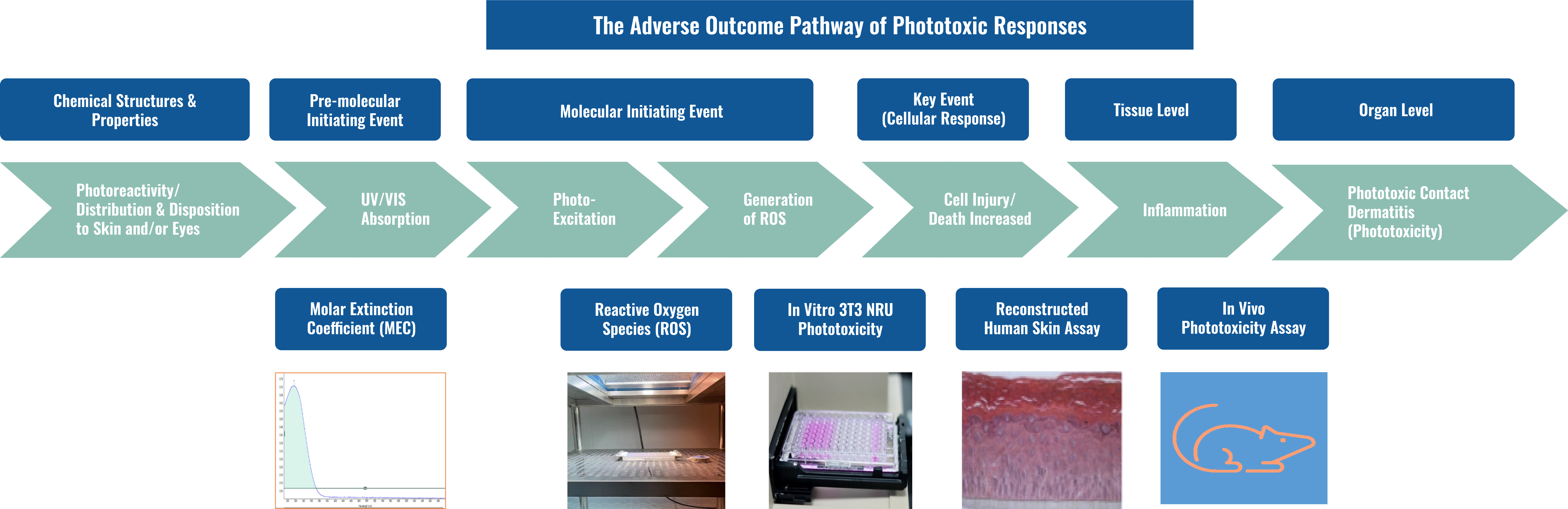
Phototoxicity Testing Capabilities
As a part of our GIVT department, WuXi AppTec also provides a comprehensive phototoxicity evaluation in collaboration with the analytical and in vivo toxicology team. The evaluation ranges from the photochemical properties, in vitro to in vivo assays.
With New Approach Methodologies (NAMs) gaining increasing attention, GIVT is keeping pace with the scientific and regulatory communities to explore the potential of NAMs (e.g., irritation/corrosion/sensitization, other NAMs adopting 2D, 3D and organoids).
GLP or Non-GLP Phototoxicity Assays
Phototoxicity assays evaluate the potential of substances to cause skin irritation under light exposure. WuXi AppTec’s standard battery of phototoxicity assays include both GLP and non-GLP assays:
- MEC
- ROS
- 3T3 NRU phototoxicity assay
- 3D RhE phototoxicity assay
- In vivo phototoxicity assay
In Vitro Toxicology Reporting
WuXi AppTec leverages an automated reporting system to draft reports and has a dedicated study director group for report checking and peer-reviewing. Our team is committed to provide high quality study data and deliver the reports timely in order to meet your timeline.
Frequently Asked Questions
What is In Vitro Toxicology Testing?
In Vitro Toxicology Testing involves evaluating the potential toxicity of substances using cells or tissues outside the body to predict their effects on humans or the environment.
How does In Vitro Testing differ from In Vivo Testing?
In Vitro Testing uses cells or tissues in controlled laboratory conditions, while In Vivo Testing involves testing on living organisms.
What are the advantages of In Vitro Toxicology Testing?
In Vitro Testing can be faster, more cost-effective, and reduce the need for animal testing while providing insights into specific mechanisms of toxicity.
What are the advantages of In Vitro Toxicology Testing?
In Vitro Testing can be faster, more cost-effective, and reduce the need for animal testing while providing insights into specific mechanisms of toxicity.
What are In Vitro Electrophysiology Assays?
In Vitro Electrophysiology Assays measure the effects of substances on electrical activity in cells, often used to assess cardiac safety or neuronal function.
How are In Vitro Electrophysiology Assays relevant to drug development?
These assays help predict potential cardiac side effects of drugs by assessing their impact on ion channels responsible for heart function.
What is an In Vitro Proarrhythmia Assay (CiPA)?
CiPA assays assess the proarrhythmic potential of drugs by integrating data from multiple assays, including electrophysiology and computational modeling.
How accurate are In Vitro Proarrhythmia Assays compared to traditional methods?
In Vitro Proarrhythmia Assays provide more mechanistic insights into drug effects on cardiac ion channels, potentially improving prediction of arrhythmia risks compared to traditional methods.
What is the purpose of phototoxicity testing?
Phototoxicity testing evaluates the potential of substances to cause toxic effects when exposed to light. This ensures safety for substances that will be exposed to light during use.
What types of cells or tissues are used in In Vitro Toxicology Testing?
Commonly used cells include human cell lines, primary cells, or engineered tissues that mimic specific organs or physiological systems.
How are regulatory agencies incorporating In Vitro Toxicology Testing into safety assessments?
Regulatory agencies increasingly accept In Vitro Testing data for hazard identification and risk assessment in drug development and chemical safety evaluations.
What are the limitations of In Vitro Toxicology Testing?
In Vitro Testing may not fully replicate the complexity of biological systems in vivo, and standardization of methods and validation are ongoing challenges.
How can In Vitro Toxicology Testing contribute to reducing animal testing?
By providing early-stage toxicity data, In Vitro Testing can reduce the reliance on animal testing, aligning with ethical and regulatory trends towards animal welfare.
What are the key considerations for choosing appropriate In Vitro Toxicology assays?
Factors include relevance to the substance being tested, the biological system being mimicked, scalability, reproducibility, and regulatory acceptance.
What advancements are being made in In Vitro Toxicology Testing technologies?
Advances include microfluidic systems, organ-on-a-chip models, and high-throughput screening techniques, enhancing predictive capabilities and efficiency.
How can companies integrate In Vitro Toxicology Testing into their drug development pipelines?
Integrating In Vitro Testing early can identify potential toxicities, inform compound optimization, and reduce the likelihood of late-stage failures in clinical trials.