Preclinical Bioanalysis
Partner with an expert team that delivers the regulated preclinical bioanalytical support your compound needs for IND submission. Our comprehensive testing programs for small and large molecules cover the full range of development needs, helping advance your compound more efficiently and effectively.
Preclinical Bioanalytical Studies
to Support IND Submission
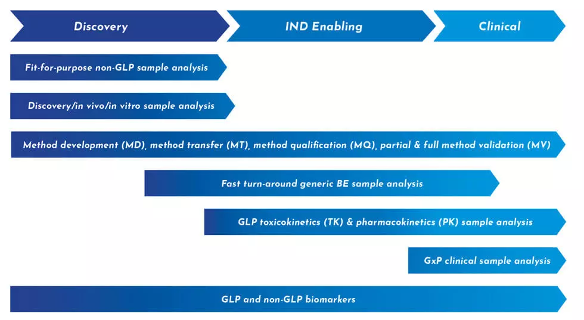
Our preclinical bioanalytical team brings decades of experience advancing both small and large molecule compounds for IND submission. As an end-to-end partner, we use insights from discovery efforts to eliminate the need to transfer a compound from one unit to another, significantly reducing timelines.
-
340+ preclinical biomarkers for small and large molecules
-
60,000 samples per month capacity
-
20,000 square feet of GLP lab space
-
State-of-the-art mass spectrometry platforms with UHPLC-MS/MS capabilities
-
Automated and semi-automated high-throughput-capable systems including Janus®, TomTec® CyBio®-SELMA\
-
21 CFR Part 11-compliant validated systems; Analyst®, MassLynx™, Watson LIMS™
Small Molecule Preclinical Bioanalysis
We offer rapid method development and optimization for small molecules, backed by deep expertise and accelerated synthesis capabilities. Our services cover a wide range of peptides and proteins from various species and sample types, using cutting-edge techniques for accurate and efficient analysis.
Rapid Method Development
-
- Peptides 2-4 weeks
- Proteins 4-6 weeks
Extensive Experience
-
- Resolve non-specific binding
- Sensitivity tune-up
- Trypsin digestion conditions
- Microelution solid phase extraction (SPE) optimization
Diversified Peptides & Proteins
-
-
Different species and matrices
-
Hybrid Immunoaffinity LC-MS
-
Fast Synthesis Capabilities
-
-
2 weeks turnaround time
-
Synthesize surrogate peptide
-
Synthesize SIL-IS
-
70+ Antibody
10+ ADC
30+ Fusion Proteins
40+ Recombinant Proteins
35+ Peptides (including Conjugated)
10+ Other
Large Molecule Preclinical Bioanalysis
We support large molecule development through a robust immunochemistry platform that covers biologics, biomarkers and vaccines. Operating out of 20,000 square feet of GLP laboratory space, our team can handle over 60,000 samples per month, and has validated more than 300 preclinical biomarker assays to support your large molecule programs.
Your Immunogenicity Assessment Journey
Biotherapeutics present many challenges in drug development compared to small molecule drugs. As a result, regulatory agencies require extensive immunogenicity testing in an IND package that, left unaddressed, can lead to clinical delays. To avoid this scenario, we help you develop custom reagents and assays that analyze key measures of the immune response, and provide expert support in executing the multi-tiered testing that adheres to regulatory agency immunogenicity guidelines.

Monoclonal
Polyclonal
Recombinant Proteins
Peptides
Custom Bioreagent Antibodies & Proteins Development
We develop custom reagents to support your candidate in the assessment of immunogenicity, PK and PD bioanalytical services. Once validated, they can rapidly analyze therapeutic candidates, to advance development efforts.
Frequently Asked Questions
What is preclinical bioanalysis?
Quantitatively measuring drugs, metabolites and biomarkers in biological samples during the preclinical stage of drug development is crucial for assessing the safety and effectiveness of potential drug candidates in animal models.
Why is preclinical bioanalysis important?
Bioanalysis conducted during the preclinical phase yields vital information about drug candidates’ pharmacokinetics, pharmacodynamics and toxicokinetics. This data is instrumental in comprehending drug candidates’ behavior in the body, influencing dosage determinations and safety assessments prior to clinical trials.
What types of samples are analyzed in preclinical bioanalysis?
Typical samples in preclinical bioanalysis comprise blood, plasma, serum, urine, tissue, and other biological fluids, which are examined to measure the levels of drugs, metabolites, and biomarkers.
What analytical techniques are used in preclinical bioanalysis?
Frequently used methods in preclinical bioanalysis encompass liquid chromatography-mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), and enzyme-linked immunosorbent assays (ELISA). These techniques provide exceptional sensitivity and specificity detecting and quantifying analytes.
How are bioanalytical methods validated?
Bioanalytical methods undergo validation in accordance with regulatory guidelines to guarantee accuracy, precision, sensitivity, specificity, reproducibility and robustness. Validation is essential to ensure the reliability and consistency of the methods’ results.
What regulatory guidelines govern preclinical bioanalysis?
Bioanalytical processes during the preclinical phase must adhere to regulatory guidelines set forth by international regulatory bodies. These guidelines establish the criteria for method validation, sample analysis and data reporting.
Can customized bioanalytical services be provided for specific projects?
Yes, tailored bioanalytical services can be customized to fulfill the specific requirements of various projects. This encompasses developing and validating unique analytical methods to address particular research inquiries.
How long does it typically take to complete a preclinical bioanalysis study?
The duration of a preclinical bioanalysis study is determined by the project’s complexity, sample quantity and the analytical methods needed. Timelines are typically established and confirmed during the project planning phase.
What is the focus of WuXi AppTec's preclinical bioanalysis services?
WuXi AppTec specializes in providing regulated preclinical bioanalytical support for small and large molecule drugs, focusing on the needs of IND submissions.
What technologies does WuXi AppTec use for preclinical bioanalysis?
Our facilities employ LC-MS/MS for peptide and protein quantitation and immunochemistry platforms for large molecule products, including biologics, biomarkers, and vaccines.
How does WuXi AppTec support immunogenicity testing?
WuXi AppTec offers comprehensive immunogenicity assessments adhering to all major regulatory agency guidelines, including developing custom reagents and assays.
What custom bioreagent development services does WuXi AppTec offer?
WuXi AppTec develops custom bioreagents such as monoclonal and polyclonal antibodies, recombinant proteins, and peptides to support bioanalytical assessments.