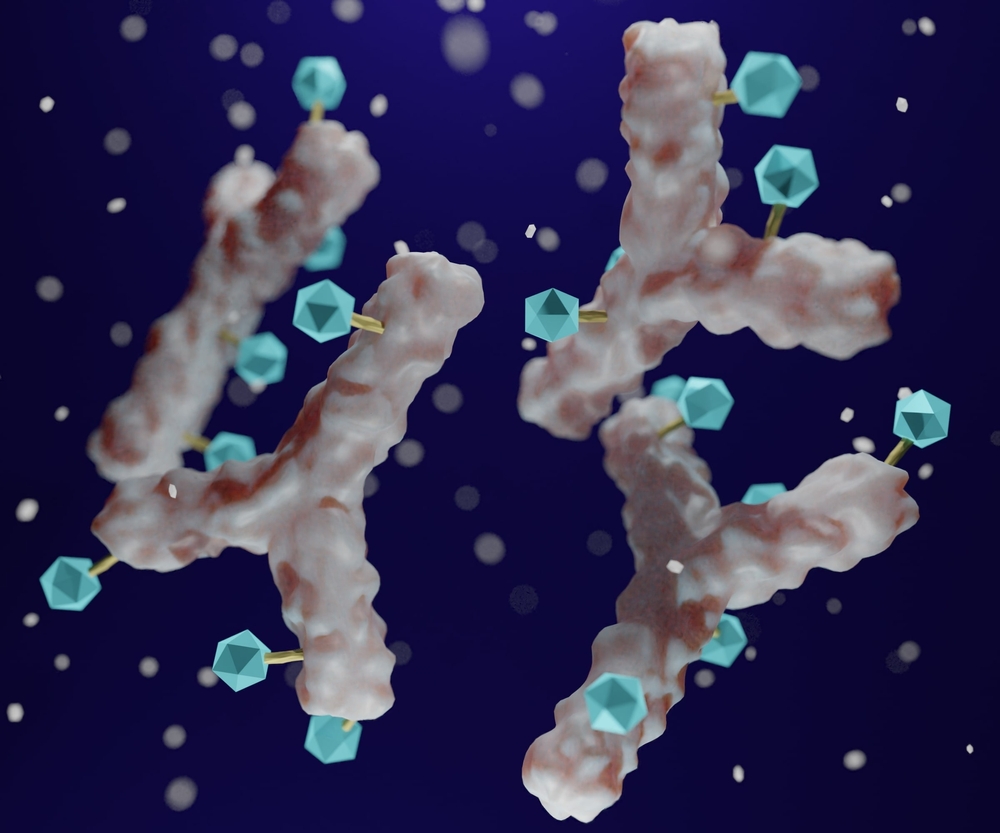
Cancer is a leading global health concern, accounting for approximately 10 million deaths in 2020, according to the World Health Organization’s International Agency for Research on Cancer. Traditional chemotherapy, which uses cytotoxic drugs like DNA base analogs (e.g., 5-fluorouracil), DNA-interacting agents (e.g., actinomycin D), antimetabolites (e.g., methotrexate), and tubulin inhibitors (e.g., paclitaxel), has been the primary treatment for decades. However, these treatments often have a narrow therapeutic window and can cause significant side effects due to non-specific targeting.
The introduction of monoclonal antibodies revolutionized cancer therapy by allowing for targeted treatment of tumor surface antigens. Despite this advancement, monoclonal antibodies alone are sometimes insufficient due to limited cancer cell death compared to traditional chemotherapy. To address this, Antibody-Drug Conjugates (ADCs) have been developed. ADCs combine the specificity of monoclonal antibodies with the potency of cytotoxic agents, offering improved efficacy and reduced systemic toxicity.
ADC Composition
ADCs consist of three key components:
- Antibodies: These can be chimeric, fragments, or bispecific antibodies that specifically target cancer cells.
- Linkers: These are the chemical bridges that connect the antibody to the cytotoxic payload. Linkers can be cleavable (using differences in the tumor microenvironment) or non-cleavable, with each type influencing the release mechanism of the payload.
- Cytotoxic Payloads: These small molecule drugs target cancer cells by inserting into DNA or inhibiting microtubule formation, leading to cell death.
ADC Mechanism of Action
The mechanism of action involves the ADC binding to specific tumor antigens, internalization by the cancer cells, and release of the cytotoxic payload, which induces apoptosis.
- Binding to Specific Tumor Antigens: ADCs are designed with monoclonal antibodies that can specifically recognize and bind to antigens on the surface of cancer cells. These antigens are typically overexpressed or uniquely present on tumor cells, making them ideal targets for therapy. The antibody specificity ensures that the ADC preferentially binds to cancer cells, sparing most normal cells.
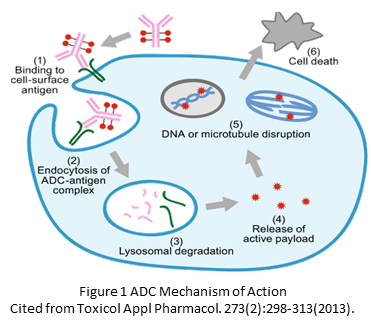
2. Internalization by Cancer Cells: Once the ADC binds to the tumor antigen, the complex is internalized into the cancer cell through endocytosis. This is a critical step, as the internalization allows the ADC to enter the cell and reach the intracellular compartments where it can exert its effects.
3. Release of the Cytotoxic Payload: Inside the cancer cell, the ADC is transported to a specialized compartment called the endosome, which later fuses with lysosomes. The acidic environment and enzymatic activity within the lysosome cause the ADC to be degraded, releasing the cytotoxic drug (payload) linked to the antibody. This payload is typically a highly potent chemotherapeutic agent designed to kill the cancer cell.
4. Induction of Apoptosis: The released cytotoxic drug exerts its lethal effect by interfering with critical cellular processes. For example, it might damage the DNA, inhibit microtubule function, or interfere with protein synthesis. These disruptions lead to the activation of cellular pathways that induce apoptosis or programmed cell death. Apoptosis is a controlled way for the cell to die, preventing the release of potentially harmful substances that could damage surrounding tissue.
Bioanalytical Challenges in ADC Development
Analyzing ADCs presents several challenges due to their complex structure and diverse components:
- Component Variability: The heterogeneity of ADCs at different time points complicates the development of standardized bioanalytical methods.
- Differing Drug-Antibody Ratios (DAR): ADCs with different DARs may show varying binding affinities, affecting the accuracy of total and free drug measurements.
- Immunogenicity: ADCs may introduce new antigenic epitopes, necessitating domain-specific immunogenicity evaluations for confirmed positive samples.
Regulatory Guidelines and Analytical Strategies
Pharmacokinetics (PK) Analysis
Regulatory guidelines recommend measuring various analytes, including the total antibody, the intact ADC, and the released small molecule payload. Because ADCs have the molecular characteristics of large and small molecule drugs, typical bioanalytical methods for each are necessary analytical tools. ELISA or MSD platforms are often used to detect the PK of total antibodies and ADC drugs. For PK detection of total antibodies, coating antigen or anti-ID (idiotype) antibodies are mainly used to capture total antibodies, followed by appropriate enzyme-labeled detection antibodies (such as anti-Fc antibody or anti-ID (idiotype) antibody) for quantitative detection. For the PK detection of ADC drugs, an anti-payload drug antibody is used to capture the ADC. Then, appropriate enzyme-labeled detection antibodies (such as anti-Fc or anti-ID (idiotype)) are selected for quantitative detection. An LC-MS platform is usually used to detect payload-related PK analysis.
Anti-Drug Antibody (ADA) Analysis
To detect anti-drug antibodies (ADAs), regulatory guidelines recommend using a bridging assay format with biotin-labeled ADCs as capture reagents and detection reagents like digoxin-labeled or ruthenium-labeled ADCs. This comprehensive process includes preliminary screening, confirmatory testing, neutralizing antibody analysis, and domain-specific evaluations as necessary.
The ADA analytical method predominantly employs the bridging approach on the MSD platform. In this method, biotin-labeled ADCs serve as the capture reagents, while digoxin (Dig) or ruthenium-labeled ADC drugs act as the detection reagents.
The primary goals of ADA sample analysis are to conduct initial screening, perform confirmatory testing on samples that test positive in the preliminary screening, and carry out neutralizing antibody analysis on samples confirmed to be positive. Given that ADAs can target different domains of the ADC, including the antibody moiety, linker, payload moiety, or the entire complex, it’s crucial to conduct domain-specific evaluations on confirmed positive samples to assess immunogenicity accurately.
For more information on ADC PK and ADA analyses, watch our video presentation, Bioanalysis of Antibody Drug Conjugates.
Neutralizing Antibody (NAb) Analysis
For NAbs, regulatory guidelines recommend cell-based assays to accurately reflect in vivo conditions. However, non-cell-based methods can be used when appropriate cell lines are unavailable, or matrix effects are insurmountable.
Positive samples for ADAs usually require analysis for neutralizing antibodies to the Nab. Regulatory authorities require cell-based in vitro functional assays to determine neutralizing antibodies. They should reflect the in vivo state. Finding appropriate cell lines is difficult, with significant matrix effects and great interference. Non-cell-based methods can be used where relevant cell lines are difficult to obtain, or matrix effects cannot be resolved.
A Final Word
The development of ADCs represents a significant advancement in cancer therapy by combining the targeting capabilities of monoclonal antibodies with the potency of cytotoxic drugs. The complexity of ADCs, comprising antibodies, linkers, and cytotoxic payloads, requires specialized bioanalytical strategies to ensure their safety and efficacy. This includes precise pharmacokinetic analysis, anti-drug antibody assessments, and neutralizing antibody testing, all adhering to stringent regulatory guidelines. By overcoming these bioanalytical challenges, the pharmaceutical industry can optimize ADC development, offering more targeted and effective treatments with reduced systemic toxicity. The continuous evolution of these strategies will play a crucial role in enhancing the therapeutic landscape for cancer patients, providing hope for more personalized and less invasive treatment options.
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the fourth consecutive year in 2024 and its open-access platform is enabling around 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that “every drug can be made and every disease can be treated.”