Single Dose Acute Toxicity Testing
Single dose acute toxicity studies help establish the maximum tolerated dose (MTD) for further testing. As an experienced partner in general toxicology testing, we can help you build a toxicology program that includes the right single dose acute toxicity studies that meets regulatory guidelines and your timeline’s milestones.
On Time Reporting
Environmentally Controlled Animal Rooms
Safety Assessment Studies Conducted/Year
Single Dose Toxicity Testing
for Your New Drug Candidate
Single dose acute toxicity studies help researchers identify potential adverse effects of a new drug candidate by exposing animals to a single high dose of a substance. This study is often used to determine the maximum tolerated dose (MTD), as well as identify potential target organs affected by the compound.
Single Dose Acute Toxicity Study Species & Routes of Administration
Our experienced team can help you design single dose acute toxicity studies with the right toxicological species, dose level, routes of administration, analyses, and regulatory support – for all stages of the drug development continuum.
Species
-
- Canine
- Mouse
- Rat
- Rabbit
- NHP
Routes of Administration
-
- Intravenous (bolus, infusion)
- Oral (gavage, capsule) and nasogastric (for NHPs)
- Topical
- Intramuscular
- Subcutaneous
- Intradermal
- Ocular (including systemic, topical, subconjunctival, intravitreal, sub-retinal and retrobulbar)
- Implant
- Other (upon request)
-
- Intrathecal
- Intra-articular
- Intraperitoneal
- Intra-arterial
-
Single Dose Acute Toxicity Data
Single dose toxicity studies generate data that describe the relationship between dose and toxicity. Gaining an understanding of the range of toxic and secondary pharmaceutical effects can help researchers select doses for repeat dose chronic toxicity studies in animals, which evaluate the effects of repeat administration over a defined period of time.
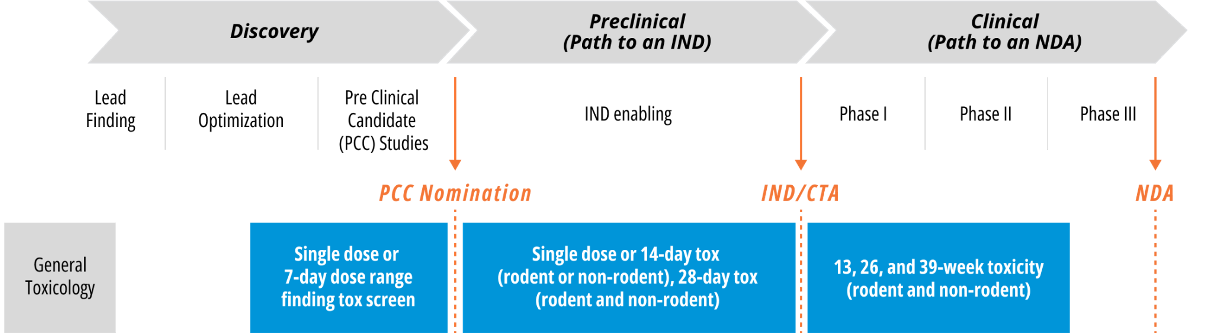
Single Dose Acute Toxicity in Drug Development
Single dose studies are critical parts of the drug development spectrum. In addition to serving toxicology screening purposes, single dose acute toxicity studies conducted under GLP conditions can be the pivotal, IND-enabling study when clinical plans require only a single dose.
Related Resources About
Single Dose Acute Toxicity Testing
Frequently Asked Questions
What are single dose acute toxicity studies?
Single dose acute toxicity studies test the safety of a new drug candidate by exposing animals to a single dose of the compound and monitoring for signs of toxicity over the course of 14 days. According to the FDA’s guidance for the industry on acute toxicity testing, the drug compound is administered in one or more doses during a period not exceeding 24 hours.
Why are single dose acute toxicity studies necessary?
Single dose acute toxicity studies are important to investigate the potential toxic effects of a compound and establish the maximum tolerated dose (MTD) for further toxicological evaluation.
How do single dose acute toxicity studies add value to my testing program?
Single dose acute toxicity studies help you identify the doses for repeat dose studies, which evaluate the effects of repeat administration over a defined period of time.
What kind of data do I get from single dose acute toxicity studies?
The data obtained from single dose studies will help you determine your doses for repeat dose studies based on an understanding of the range of toxic and secondary pharmaceutical effects observed.


