Antibody-drug conjugates (ADCs) are offering hope to cancer patients worldwide. This novel treatment combines the precision targeting of antibodies with the cancer cell-killing power of cytotoxins, delivering treatments directly to tumors and minimizing damage to healthy tissues.
The development of ADCs requires rigorous bioanalysis to understand their metabolism and toxicity. ADCs are complex drugs, and sophisticated analysis ensures researchers can quantify their properties accurately. This involves measuring total antibodies, coupled antibodies, and free payloads to establish the pharmacokinetics and pharmacodynamics of drug candidates.
The two primary analytical methods used for ADC characterization are Ligand Binding Assays (LBAs) and Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). LBA is usually used to measure the total and coupled antibodies, and LC-MS/MS offers a detailed analysis of bound and unbound cytotoxins.
LC-MS/MS: One sample for a complete component analysis
The LC-MS/MS method is used to quantitatively analyze free small-molecule cytotoxins and their conjugates with antibodies and for the comprehensive bioanalysis of ADC drugs. This method uses specific immune capture and enzymatic hydrolysis processes to ensure excellent specificity. The platform allows for the simultaneous detection of total antibodies, antibody conjugates, coupled drugs, and free drugs, all using the same sample.
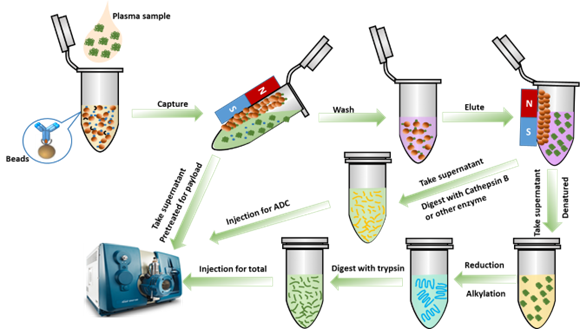
Figure 1. ADC LC-MS/MS platform analysis flow
The process obtains the supernatant, which is used to detect free cytotoxins post-immune capture. The antibody fraction is then separated into two parts to analyze the coupled and total antibodies. When analyzing free cytotoxins, researchers require two critical characteristics:
- Sensitivity: Because ADCs are so targeted, the concentration of free toxins in the plasma is low. This means a low limit of quantitation is necessary. When the regular protein precipitation approach is not able to deliver the required sensitivity, more sophisticated methods like liquid-liquid extraction (LLE), solid-phase extraction (SPE), or supported liquid extraction (SLE) can improve assay performance.
- Stability: The stability of the linker connecting the antibody and the toxin in plasma is crucial to determine the concentration of the free toxin. Stability testing needs to assess the strength of the toxin in the biological matrix and establish if the ADC releases free toxins during sample storage and pre-treatment.
Establishing DAR value
Metabolic processes ensure that the loading of small molecule drugs on an antibody in ADCs shrinks over time, affecting the drug-to-antibody ratio (DAR). Researchers need to monitor DAR changes over time to understand the safety and efficacy of ADCs. Ideally, an ADC should release the cytotoxin at the target organ rather than into the blood circulation. This minimizes the toxicity and ensures the maximum therapeutic efficacy.
High-resolution Mass Spectrometry Quadrupole-Time of Flight (HRMS-Q-TOF) analysis offers benefits over triple quadrupole mass spectrometry methods, as it has superior resolution, specificity, and accuracy. This is especially true as ADCs with different DARs have varying molecular masses. However, HRMS-Q-TOF does have sensitivity issues and can be limited.
There are several approaches to DAR determination. One involves analyzing the complete ADC molecule’s DAR, which requires steps such as immune capture and deglycosylation before high-resolution mass spectrometric analysis. This directly reveals the toxin’s attachment to the ADC molecule. For toxins linked specifically to light or heavy chains, alternative strategies can involve specific reagents or enzymatic hydrolysis to analyze ADC subunits; this offers enhanced sensitivity. Researchers need to tailor these approaches to individual cases to achieve accurate analysis.
Choosing a platform
LBA and LC-MS/MS platforms offer distinct reagent sensitivity, specificity, and throughput advantages. The LBA platform requires extra attention to preparing specific reagents, which influences the specificity and development time. However, it excels in sensitivity and achieves picogram per milliliter quantitation.
LC-MS/MS analyzes antibodies through characteristic peptide fragments and doesn’t require specific reagents. This means lower cost and shorter development times compared to LBA. LC-MS/MS can also analyze various ADC forms in a single sample.
Preparing specific reagents can be time-consuming, and sometimes their specificity is suboptimal. LC-MS/MS can allow for rapid method development, validation, and sample analysis. It can also analyze samples within around six weeks, reducing costs as it negates the expensive preparation of reagents. LC-MS/MS also allows researchers to determine the concentrations of all sample components concurrently, which leads to more comparable data.
A final word
The development of mass spectrometry technology will further enhance the sensitivity of LC-MS/MS analysis, expanding its application range. The platform already has unique advantages, making it an obvious choice for researchers working on ADC drugs.
Choosing an analysis platform depends on the specific requirements of the study, and there are still examples where LBA is better suited. However, LC-MS/MS is the best option if time and cost are critical factors. If drug developers lack the required expertise or capacity to perform these analyses, it’s highly recommended they work with a trusted lab partner.
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the fourth consecutive year in 2024 and its open-access platform is enabling around 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that “every drug can be made and every disease can be treated.”