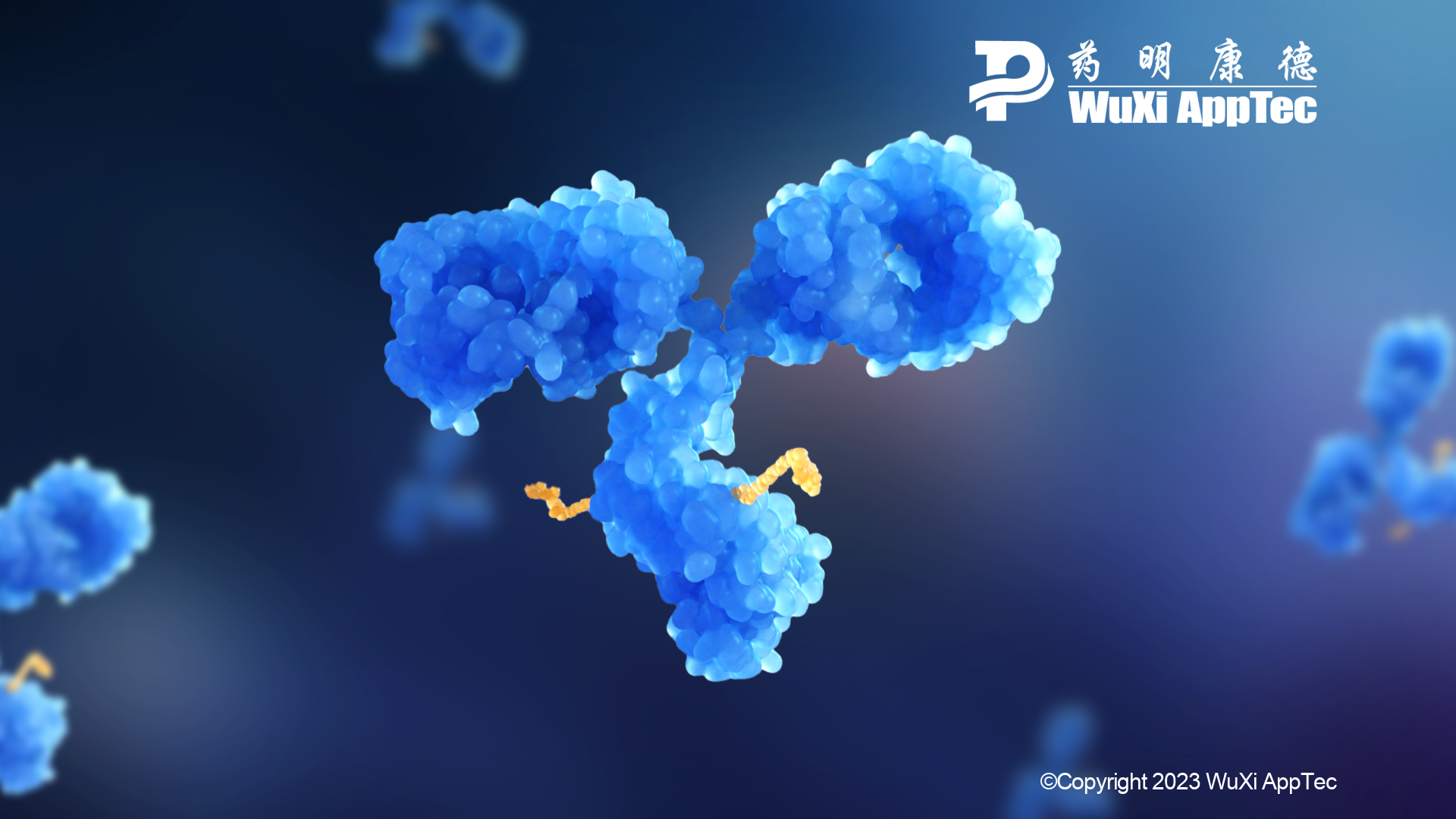
Antibody-drug conjugates (ADCs) target and destroy cancer cells like few available therapies. Highly potent and laser-focused, these tools have been around since 2000; currently, just 14 of them have been approved for use.
ADCs are made up of a monoclonal antibody, a cytotoxic payload and a chemical linker. They are very effective cancer killers but their structures create investigational challenges. ADCs’ pharmacokinetic (PK) profiles also reveal inherent challenges with stability and toxicity.
Regardless of development phase, investigators must employ both small- and large-molecule approaches to monitor an ADC’s PK and biotransformation. We’ll discuss a few bioanalytical approaches and offer recommendations for drug sponsors developing ADCs.
ADC Investigations: An Introduction
ADC bioanalysis helps investigators understand the drug’s structure, which will ultimately form the basis of the study strategy.
ADCs contain cleavable and non-cleavable linkers. The cleavable linkers use the inherent properties of target cells to selectively release payload from ADCs. Non-cleavable linkers act differently. They release their payload by breaking down or degrading antibodies. The released payloads then bind to peptide chains or amino acids.
Understanding payload release and related components is critical for ADC investigations. In target tissues, payload release determines an ADC’s efficacy, but in non-target areas, it determines the drug’s toxicity.
Establishing the drug-antibody ratio (DAR) is another critical step. The DAR is the number of payloads conjugated to each antibody, which determines whether an ADC is safe and effective. For FDA-approved ADCs, the DAR range is typically 3-4. This means three or four payloads conjugate to a single antibody.
A high DAR means a drug is released from the body more slowly, which can build up over time (i.e., aggregate) and lead to increased toxicity.A high DAR does not signify a poor ADC, it just means the payload and linker design must be chosen specifically to match the DAR.
Given ADCs’ complexities, monitoring active and deactivated drugs simultaneously creates bioanalytical challenges. But specific analytical tools can help provide the appropriate sensitivity, specificity and toxicity answers investigators seek. The four most common bioanalytical platforms include:
- Ligand-binding assays (LBAs)
- Hybrid LBA & liquid chromatography–mass spectrometry (LC-MS)
- Triple quadrupole based traditional LC-MS
- Liquid chromatography/high-resolution mass spectrometry (LC/HRMS)
The platform you use depends on your study’s bioanalytical goals. LBA may be the first choice in antibody analysis, but LC/HRMS or LBA-LC-MS are better tools for capturing DAR and biotransformation data. A trusted lab testing partner can help determine the most effective platform.
Recommendations for ADC Bioanalysis
Establishing priorities during ADC investigations is how you make sure your program stays on track. During discovery, drug efficacy is the chief concern, and so DMPK analyses are the priority. These studies connect the ADC’s efficacy and potential toxicity. This helps investigators decide on the linkage, payload and antibody design.
A drug candidate’s ADME properties (i.e., absorption, distribution, metabolism, and excretion) combined with PK data, will determine its stability, efficacy and safety. Hybrid LBA & LC/HRMS can be used to monitor intact ADCs, which is the most effective way to understand drug stability. Establishing a complete picture of the ADC, including DAR and biotransformation data, will require time and resources, but it will also provide valuable data. Put simply, this information will ultimately determine if your ADC moves forward or gets scrapped.
ADCs are an exciting but emerging field for regulators. For example, intact protein bioanalysis is a unique and popular assay, but there is little regulatory guidance around its methodology or validation. However, that may change as ADCs evolve and additional use cases emerge.
The Final Word
Developers and sponsors like ADCs’ potential as highly effective cancer-killing therapies. They are complex drugs that require small- and large-molecule studies to ensure stability, efficacy and safety. In truth, ADC bioanalysis requires an integrated strategy to be truly effective.
Researching and developing safe, effective ADCs relies more upon the investigator’s skills, knowledge, and expertise than the specific assays or platforms used. Drug developers without the time, resources or expertise to conduct ADC investigations in-house should consider working with a testing partner.
Talk to an expert about your upcoming project to see how we can help.
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the fourth consecutive year in 2024 and its open-access platform is enabling around 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that “every drug can be made and every disease can be treated.”