PROteolysis TArgeting Chimera (PROTAC)* drugs hold enormous potential. No PROTAC drugs have earned FDA approval yet, but they are being widely explored across industry and academia for an ever-expanding range of treatments.
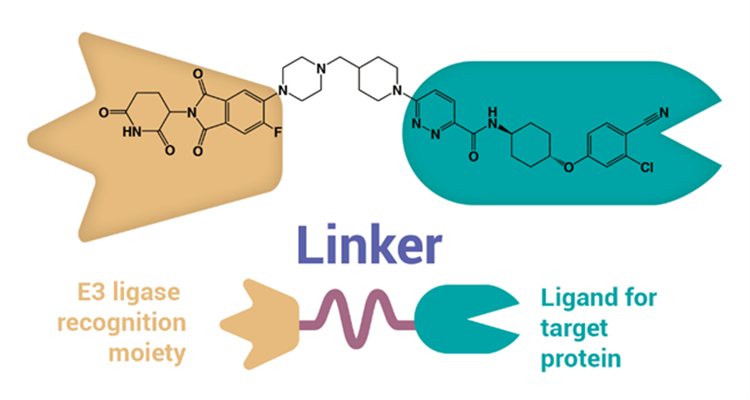
Part of PROTACs’ appeal is in their structure and mechanism of action. The molecules consist of three components: a ligand binding to target protein, another ligand binding to E3 ligase, and a linker between the two ligands. While conventional small molecules block target proteins via direct inhibition, PROTACs bind specifically to target proteins and induce degradation using the ubiquitin-proteasome system (UPS).
This gives PROTACs two main advantages: the first is their high potency, which makes them effective at lower concentrations and extends their efficacy for a longer time. The second benefit is they can target “undruggable” diseases—some disease-causing dysfunctional proteins can be targeted by PROTACs but not routine inhibitors.
Despite clear benefits, PROTACs still come with challenges. Due to their structural features, they do not adhere to the Lipinski Rule of Five, indicating they could have poor absorption or permeation. This makes oral bioavailability in vivo very challenging.
But there are ways developers can optimize the physicochemical and pharmacokinetic properties to address PROTACs’ bioavailability shortcomings. These may affect the drugs’ efficacy but the aim in this case is to improve the pharmacokinetic properties. Here are seven ways to improve PROTACs’ oral bioavailability.
1. Take them with food
All oral medications need to be dissolved before they are absorbed in the intestine, but PROTACs suffer from poor aqueous solubility. But research has shown that the solubility of PROTACs improved in a biorelevant buffer, especially in fed state simulated intestinal fluid. The findings of the research suggested the pharmacokinetics of PROTACs may achieve a better in vivo drug exposure if the patient eats food before taking them. The clinical trial design of ARV-110 and ARV-471 showed that the clinical administration of the two PROTAC molecules was “once daily with food.”
2. Improve metabolic stability
Developers can also improve the metabolic stability of the PROTAC to increase the oral bioavailability. When a compound is absorbed in the intestine, it is firstly metabolized by the liver or intestine before entering the systemic circulation—the “first pass” metabolism. This can limit the oral absorption of most drugs, but there are multiple strategies to improve metabolic stability, such as changing linker length, altering the linker’s anchor point, using cyclic linkers, and changing the linker’s attachment site.
3. Improve cellular permeability
For drugs to be absorbed when taken orally, they need to pass through the membrane barrier of the small intestine, and to achieve intracellular protein degradation, PROTACs also need to enter target cells. Both of these processes can be improved by optimizing the linker structure.
4. Choose smaller E3 ligands
PROTACs’ properties are closely determined by the types of E3 ligases. There are many different kinds applied to PROTACs but the most frequently used are CRBN and VHL. The latter type is unlikely to have high oral exposure, but CRBN-targeted PROTACs have a smaller molecular weight and are considered more “oral drug-like.” The first two PROTAC molecules to enter clinical phase II utilize the CRBN E3 ligase. It is expected that other ligands with smaller molecular weight will be found.
5. Introduce intramolecular hydrogen bonds
PROTACs generally have high polarity and many rotatable bonds, which doesn’t make a great structure to pass the lipid bilayer of the cell membrane. Recent studies have found that the formation of intramolecular hydrogen bonds can facilitate cell permeability in PROTACs by reducing their molecular size and polarity. In that research, an original strip-type molecule was transformed into a “ball.” This is a shortcut but it can be challenging to accomplish.
6. Use a prodrug strategy
A prodrug is obtained by modifying a pharmacologically active compound and is a common approach to improve a drug’s oral bioavailability. Prodrugs transform in vivo to release the active metabolite mainly by enzyme catalysis. It’s not yet been proven that prodrugs can be a successful tactic for PROTACs in practice. They may further increase the molecular weight of a PROTACs, which would cause more challenges. However, investigations into this are ongoing. A prodrug has been designed from a PROTAC compound by adding a lipophilic group to the CRBN ligand, and early results show an improvement in the PROTAC’s bioavailability.
7. Use molecular glues
PROTACs’ structure means they inevitably have a relatively large molecular weight. Chemists are exploring new strategies to reduce that weight and make PROTACs more “drug-like.” Molecular glues are compact molecules that lack a linker and can trigger a ternary complex like that of PROTACs. For that reason, they’re seen as a potentially promising new alternative.
A final word
PROTACs have the potential to target “undruggable” diseases, which is why it is worth putting in the work to make the most out of them. The seven tips above will help drug developers and sponsors take crucial steps forward. Greater collaboration between academia, drug developers and lab testing partners is essential to ensure PROTACs realize their full potential.
*PROTAC® is a registered trademark of Arvinas. In this article, PROTAC specifically refers to the abbreviation of PROteolysis TArgeting Chimera as therapeutic modalities.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, and Nanjing), ~1000 scientists and over ten years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1000+ global clients, and have successfully supported 600+ IND applications.
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the fourth consecutive year in 2024 and its open-access platform is enabling around 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that “every drug can be made and every disease can be treated.”