Molecule tracking via preclinical testing is a significant yet necessary investment for drug developers. The stakes are high, and it’s critical to stay apprised of your program’s progress from start to finish. You need to monitor how your compound is progressing and which studies have launched in order to plan for the next steps. Make sure your laboratory testing partner has the technical tools needed to effectively connect you to your program.
Laboratory testing partners, like WuXi AppTec, are utilizing advanced technology to help drug developers stay connected to their program and to keep them informed of their progress. Quick Tracer, the latest platform for this technology, has revolutionized how drug developers interact directly with their molecule and our laboratory technicians at any time and from nearly any location. Understanding how the program works and learning its capabilities can help you navigate the preclinical development process and plan for more accurate timelines.
The Ins & Outs: What You Need to Know
Drug development is expensive. It’s time-consuming. It’s hard work. But it’s rewarding.
Both failures and successes have propelled your molecule to this point of preclinical testing and it is vital to stay up to date on the status of each study. There is simply no room for error.
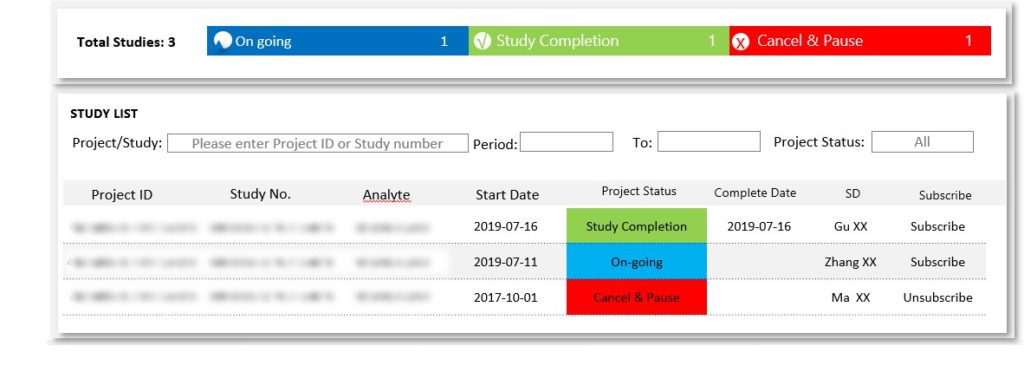
Quick Tracer allows teams to be closer to their studies, analyze results and accurately predict timing. The system presents you with a summary of all your pharmacokinetic (PK) and absorption, distribution, metabolism and excretion (ADME) studies, giving you instant access to data that helps you quickly evaluate your findings. You can also export data specific to your molecule for further review.
If your team is managing more than one program, the platform allows you to search all the studies and molecules while comparing progress. Drug development is a complicated process, but laying out the data in charts and graphs while the studies are still materializing makes it easier to manage.
In this industry, we often say, ‘communication is key,’ because of the considerable breadth of safety and efficacy data needed to support a molecule. Receiving timely notifications on your programs is a substantial benefit for drug developers, whether it be to update investors, monitor timelines or simply stay knowledgeable of the drug’s performance.
Prioritize Program Tracking
Quick Tracer’s ability to track project turnaround time (TAT), can help you prepare for downstream activities more accurately. Having the ability to closely track and share your molecule’s real-time progress will allow for efficiencies in planning that provides a significant advantage. For example, if Quick Tracer is reporting faster than anticipated movement in a PK study, drug developers can respond proactively and begin preparing for the next round of studies reducing time between tests.
Tracking platforms also provide an advantage when partnering with international laboratories. In an ever-connected global landscape, you can expand your network of partners despite distance or time zones with the support of platforms like Quick Tracer. Partnering with a laboratory testing partner that can connect you to your studies in real-time will bring you one step closer to your goals.

Working with a laboratory that prioritizes your needs is indisputable, and for many, this includes a transparent platform that reports study progress and results. Make sure you understand the capabilities and tools that laboratory testing partners provide to benefit their customers.
Learn more about WuXi App Tec’s DMPK solutions and our other state-of-the-art preclinical testing by contacting us.
Follow WuXi AppTec Laboratory Testing Division on LinkedIn for industry insights and program support.
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the fourth consecutive year in 2024 and its open-access platform is enabling around 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that “every drug can be made and every disease can be treated.”